Are you know how to Calibrate Blood Pressure Monitor Omron. Completely Omron blood pressure monitors are clinically proven accurate. They are clinically authenticated to be in the interior the following: Blood pressure: within +/- 3 mmHg or 2 percent. Pulse: within +/- 5 percent of reading.
Because your blood pressure monitor works automatically, it will need to be re-calibrated at least once every two years to be sure it is giving you accurate results. To have your automatic home monitor re-calibrated, you will need to send it back to the manufacturer.
Calibrate Blood Pressure Monitor Omron
The Omron Blood Pressure Monitor is a medical device used to measure a person’s blood pressure. Calibration of a blood pressure monitor is the process of adjusting the device to ensure that it provides accurate readings.
Calibration is typically done by the manufacturer and is necessary to ensure that the readings are accurate and reliable. Omron blood pressure monitors are calibrated to ensure that they meet the accuracy standards set by regulatory bodies such as the Association for the Advancement of Medical Instrumentation (AAMI) and the European Society of Hypertension International Protocol (ESH-IP).
If you are concerned about the accuracy of your Omron blood pressure monitor, you can contact the manufacturer or a qualified service technician to have it checked and recalibrated.
How to Calibrate Blood Pressure Monitor Omron
To get blood pressure readings, this device should be calibrated by you or your doctor with a traditional arm or wrist cuff blood pressure meter (not included). Blood pressure (BP) varies by the individual so each User must complete his/her own calibration before using the Performance Monitor <Body Check> function to measure or track your blood pressure.
Monitoring the blood pressure of a hypertension patient is one of the most effective ways to treat hypertension. Digital blood pressure monitors are an invention that helps to aid the monitoring of blood pressure for hypertension patients.
However, after comparing the accuracy of the measurements with the sphygmomanometer, the digital blood pressure monitor offers convenience benefits but sacrifices the accuracy of the measurements (de Greeff, et al, 2008).
Omron BP710N is humble to use an upper arm Blood Pressure Monitor. It will supply up to 14 readings in its recollection for a single user, allowing you to track designs of the heart. Uses Omron’s wide range cuff that will appropriate arms of 9 to 17 inches.
What’s Comprised?
1. BP710N Unit
2. Wide-range cuff (fits arms 9″ to 17″ in perimeter)
3. Instruction manual
3. Quick start guide
Topographies
1. Wide-range D-ring cuff securely provide accommodations arms from 9” to 17” in perimeter
2. One-touch process gives you an accurate reading simply with the media of a single button
3. Digital presentation is large and easy to read
4. No. 1 doctor and pharmacologist recommended home blood pressure monitor
5. Feel authorized by the accuracy with exact readings you can trust
6 Pathway your health at home with the ability to review the last 14 readings
Blood Pressure Pieces of Evidence
Your heart is your most important structure, and it’s a good idea to know how well it’s doing. With Omron home blood pressure monitors, you dependably get an accurate picture of your heart’s health.
One in three adults agonizes from high blood pressure in America. This disorder can lead to heart occurrences, strokes, kidney failure, and sightlessness. With the Omron 3 SEQUENCE upper arm monitor, you can monitor your blood pressure at home, and with the help of your doctor, take steps to reduce your risk of heart sickness. A healthy heart combined with a healthy lifestyle will help increase your probability of a long, active life.
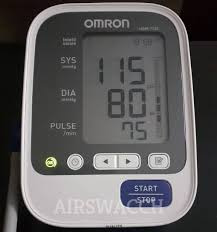
What the Numbers Mean
Empathetic your readings are easy with the Omron 3 SEQUENCE blood pressure monitor. The easy-to-read screen displays your readings in great font and labels each number with a tab along the side.
Systolic Pressure is the top number on the exhibition. Diastolic Pressure is the lowest number on the display. It’s the pressure between beats when your heart reduces. These numbers syndicate to give you your blood pressure reading (systolic over diastolic pressure).
Blood Pressure Tips
Your blood pressure numbers often change. For instance, your blood pressure in the morning can be meaningfully different than at night, when you visit a doctor’s office, or after working out.
By using an Omron blood pressure monitor at home, you’re able to detect changes in your blood pressure any time you poverty.
To ensure an accurate reading, don’t measure your vital sign within a half-hour of:
Exercising
Eating
Smoking
Drinking Alcohol
Bathing
Be sure to home the Omron blood pressure arm cuff on your left-hand arm and sit silently for a few minutes with both feet level on the floor. Wrap the cuff around the upper arm and rest an arm on a table so the cuff is level together with your heart. Press start and the cuff will inflate. Review your results in about 20-30 seconds.
This device gives a very accurate reading of an individual’s blood pressure but it requires training and a certain skill set. Thus it is not very convenient for the patient to do the blood pressure checking alone. This effect can raise blood pressure for hypertension patients up to 15% to 30% (Picker, et al, 1988).
Measure of Blood Pressure
Thus, a user-friendly device for the patient is necessary yet it has to have the capability to obtain accurate blood pressure measurements.
Currently, devices used out in the field (and not in a medical office) are digital blood pressure monitors, such as Omron. More read How to Calibrate Blood Pressure Monitor Omron.
However, these digital monitors don’t have a method to re-calibrate the device resulting in it being necessary to replace the devices pretty regularly in order to get an accurate reading of the blood pressure for hypertension patients. This is an obvious problem considering how it is very inefficient and not very realistic for poorer countries that cannot afford this luxury.
Experiment 1
This blood monitor was chosen for this project because the Omron brand is commonly used in hypertension treatment and blood pressure monitor regulation processes in out-of-clinic settings based on Amazon.com. More Read How to Calibrate Blood Pressure Monitor Omron.
Experiment 2
Two blood pressure settings were tested: normal blood pressure condition and hypertension blood pressure condition. The normal blood pressure condition was set at 120/80 mmHg and the hypertension blood pressure condition was set at 150/100 mmHg. 20 measurements were taken for both conditions.
Experiment 3
In order to investigate the causes of the blood pressure measurements variation between the Omron blood pressure monitor and mercury sphygmomanometer, the following experiment was conducted to verify the hypothesis that the air was retained in the arm cuff 5 Figure 2: The experiment set up for experiment 3 for Omron measurements. The Omron device used is model BP 742. causes the variation.
Tips How to Calibrate Blood Pressure Monitor Omron
This could cause possible air pressure detection issues for the device since the device uses mostly pressure resistor sensors (US2007/00381278A1). Thus, from a professional standpoint of view, they should aware of the arm cuff deflation completion before each usage of the device.
Omron Automatic Blood Pressure Monitor
Figure 2 shows the experimental setup for experiment 3 for the Omron automatic blood pressure monitor. Figure 3 shows the experimental setup for experiment 3 for the mercury sphygmomanometer.
(FAQs)
Q. Can Omron Blood Pressure Monitor be calibrated?
A. Omron Automatic Blood Pressure Monitors do not necessitate calibration. There will be no charge if within the guarantee period.
Q. Which Omron Blood Pressure Monitor is most accurate?
A. We’ve even provided our preferred Omron models, counting our top choice, the Omron 10 Series Wireless Blood Pressure Monitor, which takes three readings at one-minute intermissions and provides an average for greater correctness.
Q. Can home blood pressure monitors give false readings?
A. But home blood pressure monitors aren’t always as precise as they should be. “Home blood pressure monitors may be imprecise in 5% to 15% of patients, depending on the verge for accuracy used.
Conclusion
Omron HEM-7130 is a compact, fully automatic blood pressure monitor that possibilities accurateness, comfort, and affluence of use. Omron HEM-7130 Blood Pressure Monitor provides comfortable upper arm blood pressure measurement with Improved IntelliSense Technology. Our Improved IntelliSense Technology automatically puts on the right amount of pressure for fast, accurate, and more comfortable measurements.
